Process Development Services
Focusing on Optimisation of Your Bioprocess
Working with us you can expect extensive up- and downstream process development expertise shaped by multidisciplinary projects. We find the most appropriate cell line for your recombinant protein, optimize productivity, and establish a high-yield purification process while constantly monitoring quality. To achieve this, we use a broad portfolio of analytical methods, acquired over years on miscellaneous proteins.
Our Core Competencies in Process Development Services:
- USP and DSP – Bioprocess development services ready for tech-transfer and scale-up to pilot and production level
- Bioprocess engineering and optimization of existing processes
- Considering purity demands in accordance with GMP/ICH guidelines and control strategy implementation
- Application of design of experiment (DoE), robustness tests
- Formulation development
- Stability studies under R&D
- Development of analytical assays
- Protein modification

Upstream Process Development
Upstream process development at FyoniBio offers biotechnological contract research and development based on mammalian cell culture systems (GlycoExpress®, CHOnamite®).
Besides stand-alone fed-batch and perfusion process development we develop intensified fed-batch processes.
We are equipped with state-of-the-art bioreactor technologies from 5 ml to 10 L reactor volume:
- Clone screening at 10-15 mL scale in batch, fed-batch or perfusion mode (Sartorius Ambr 15)
- Upstream process development and optimization at 12 mL to 10 L scale for batch, fed-batch, intensified fed-batch or perfusion (Xcell™ ATF or Centritech®) operation

Over all stages of process development, we offer to include our analytical services & bioassays department for in depth quality determination of products and intermediates. Trust our experience in tech transfer to US and European GMP facilities to move your protein forward once the process is complete:
- Track record of successful process development for mAbs (including defucosylatad Abs), bispecifics / antibody fragments / fusion proteins, enzymes / hormones, blood factors and other glyco-optimized or difficult-to-express proteins
- High yield expression combined with high reproducibility and scalability
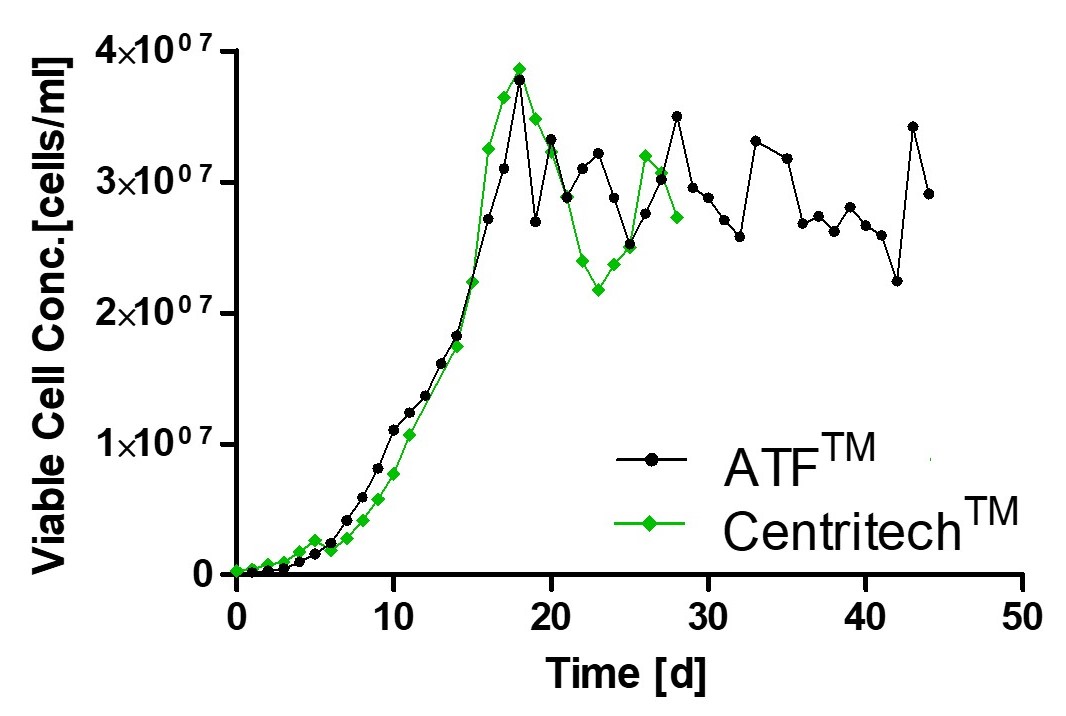
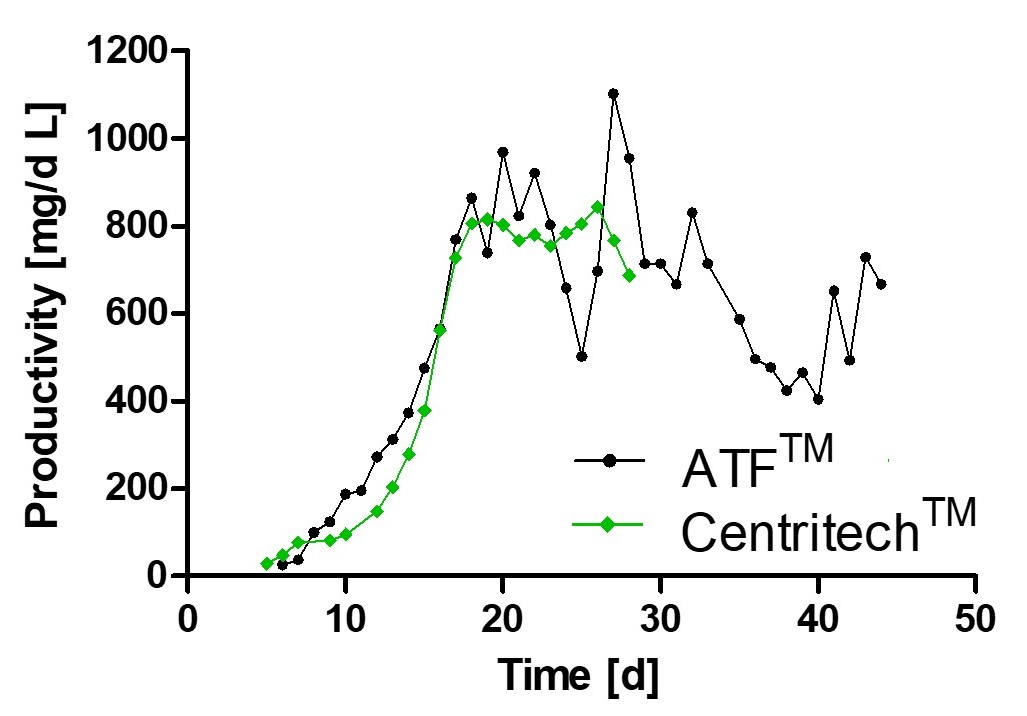
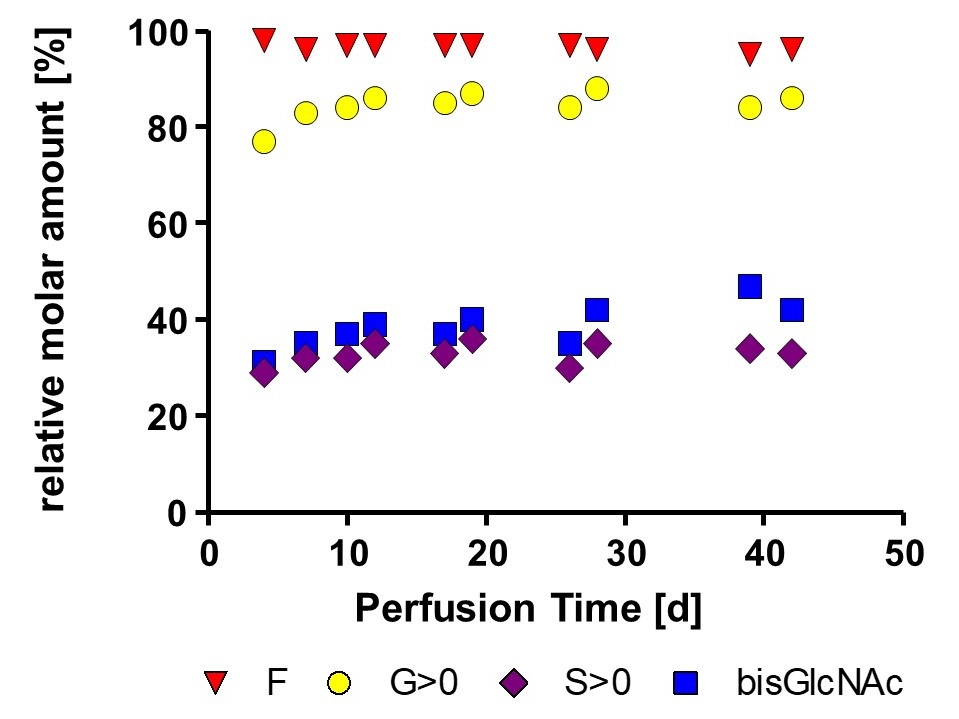
Using a Perfusion Process in GEX® for Consistent Quality of your Product
Monitoring of viable cell density and productivity showed highly comparable results using different separation devices thereby underlining process consistency.
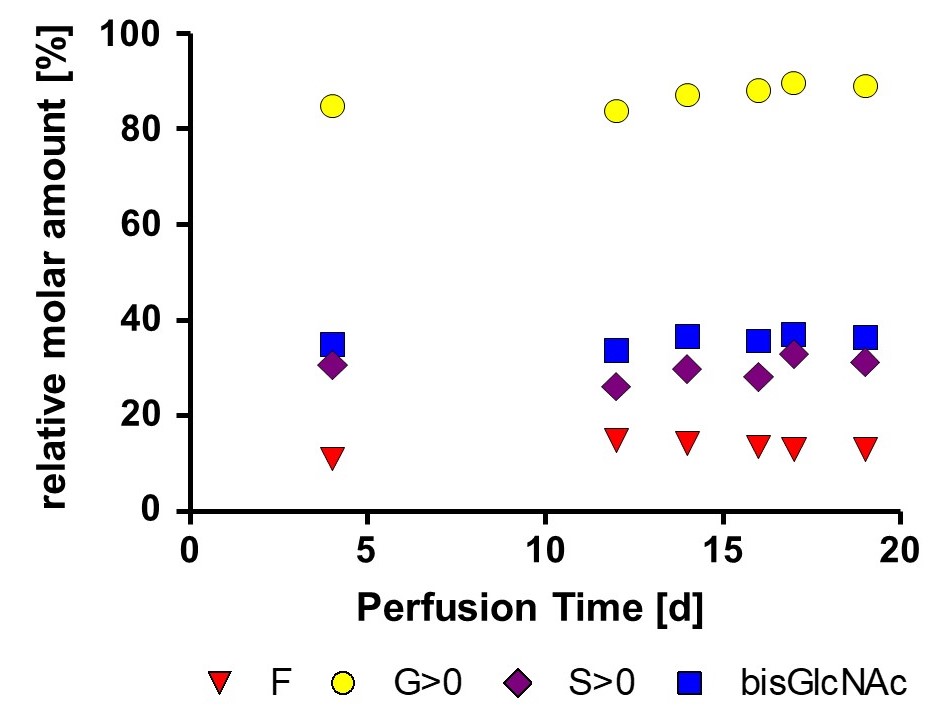
Long term stability of glycan profil was detected for a low fucose optimized mAb and a non-optimized mAb. Data was obtained from 1000 L working volume runs at GMP facility.
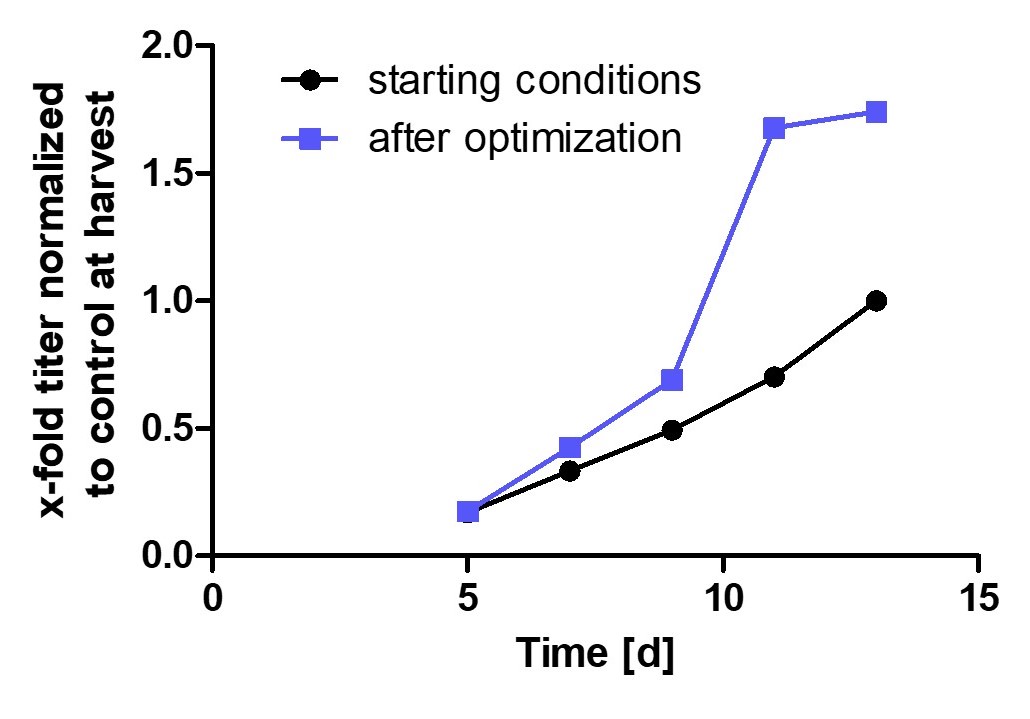
Process Optimization – Case Study
Customer case study of a fed-batch process aiming for increasing process productivity while improving unwanted glycan content on protein. Excerpt from 48 simultaneous bioreactor runs at 15mL final volume.
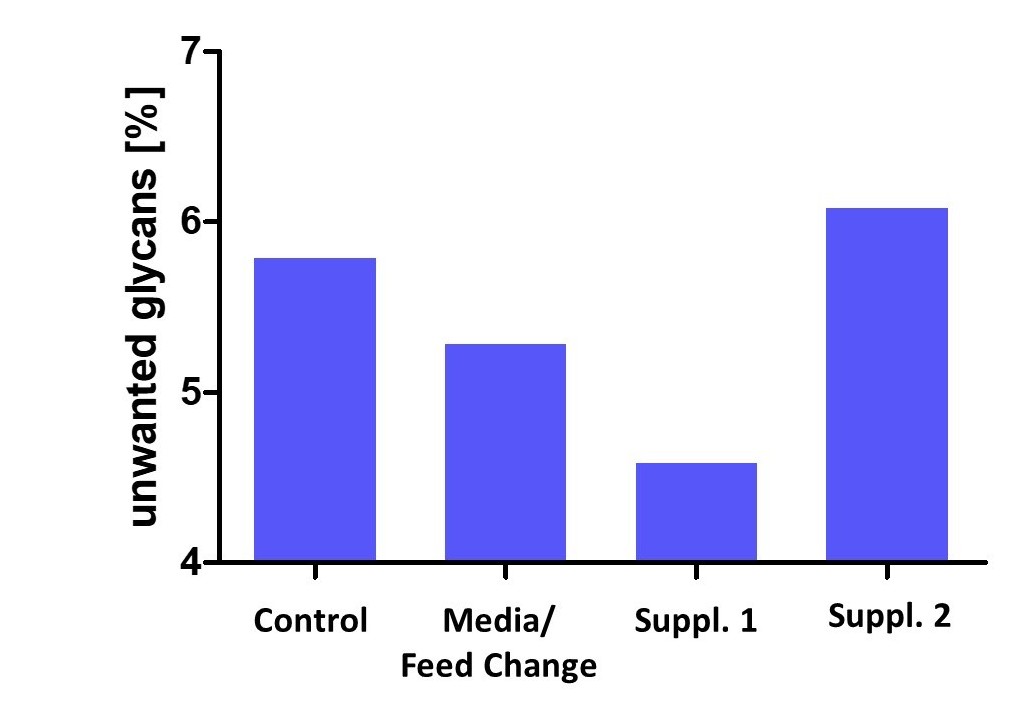
Downstream Process Development
During development of downstream processes (DSP) structural and functional parameters of your recombinant protein must be retained while simultaneously all potential impurities are removed.
We perform method development for protein purification processes including
- Chromatographic purification development (capture, polishing) including e.g.
- Affinity
- Ion exchange
- Hydrophobic interaction
- Mixed mode
- Filtration, buffer exchange and concentrating (cell separation and TFF/diafiltration development)

As a result we achieve a robust and efficient process for the removal of host cell proteins (HCP), culture medium components, and host cell DNA as critical process related impurities.
Special emphasis is given on the prevention of protein aggregation and fragmentation which might occur during production and purification.
Development of Analytical Methods
A broad spectrum of analytical methods is available to monitor the product quality resulting from up- and downstream processes including:
- HPLC (e.g., SEC, WCX, WAX, RP, HIC)
- ELISA, SDS-PAGE, Western Blot, IEF
- Protein-specific functional assays (activation, clotting, binding etc.)

Additionally, FyoniBio offers a comprehensive portfolio of analytical services & bioassays to characterize your protein, e.g., affinity, post translational modifications (PTMs), N- and O-glycans. Of course, every assay is customized or can be newly developed from scratch.
Formulation Development as a Part of Process Development Services at FyoniBio
During long-term storage of proteins, degradation or loss of functionality can occur. Most prominent reasons are aggregation, fragmentation, deamidation and oxidation.
FyoniBio uses accelerated stress studies for liquid or lyophilized storage buffers to optimize the stability of your protein. As additional tool we use buffer screening by the means of changes in intrinsic or dye fluorescence.
Protein Modification
Do you need modified proteins? We conjugate your molecule with toxins (ADC), streptavidin, biotin, fluorescent label or else. Or do you want to create your own affinity chromatography matrix? We couple your protein to NHS-activated resin or magnetic beads.
Need more information about our Process Development Services?
Frequently Asked Questions (FAQ) for Process Development Services
At FyoniBio, upstream and downstream process development can be performed and adapted to provide a customized solution for each project. From upstream development several small-scale models are available to mimic large scale production processes. From simple shake flask and SPINtube models up to well monitored AMBR15 small scale bioreactors, optimization strategies for fed-batch and perfusion processes can be performed.
The FyoniBio staff has many years of experience in process development and optimization for antibodies, bispecific antibodies, blood factors and other glycoproteins of their internal pipeline as well as of customer projects. Starting from affinity capture, one or two polishing steps using ionic, hydrophobic or mixed mode chromatography lead to very pure products with lowest HCP, HCD, and aggregate content. In addition, formulation development and stability studies find buffers suitable for long-term storage of the protein.
During a perfusion process, the product is constantly removed from the bioreactor while fresh media is fed. In contrast, in a fed-batch process the product stays over 14-17 days in a bioreactor at cell cultivation conditions (37 °C, high shear forces). If products are unstable over fed-batch cultivation time, perfusion processes might be the process of choice as harvested supernatant can be directly stored at the required storage conditions. Apart from the fact of instability, posttranslational modifications can be kept constant over the time period the perfusion process is run by continuously holding cells in a constant state.
Protein purification processes consist of several steps. Optimally, an affinity chromatography resin is available, such as Protein A for immunoglobulins, since it results in very pure target protein. One or two polishing steps follow, which can be ion exchangers, hydrophobic interaction or mixed mode resins. Often the buffers have to be exchanged or adapted between steps.
The goal of each downstream process is to get rid of host-cell proteins and DNA, eliminate possible virus contamination, and deplete aggregated or inactive target protein. Preserving functionality must always be kept in mind.
For classical IgG1 molecules a platform downstream process is available which is adjusted to the individual mAb in a short and cost effective manner.
A protein is a very complex molecule. Therefore, several methods have to be used to fully characterize it. Methods concerning amino acid composition, glycosylation or other post-translational modifications are described in the in the section about mass spectrometry services. However, intact mass, aggregates, fragments, net charge, identity and isoforms can also be detected by other methods:
– SDS-PAGE separates proteins with respect to their mass. Aggregates, fragments, and impurities are visible. With respect to a reference or a calibrator, identity and integrity can be assessed.
– Western Blotting can identify protein bands in an SDS-gel by transferring them to a membrane and staining with suitable binding partners.
– SEC is a chromatography method which separates proteins of different mass.
– Weak ion exchangers or IEF gels can separate differently charged proteins.
– Affinity to possible binding partners (if any), by DRX2 or Octet technology.
The biochemical and biophysical protein characterization is complimented by protein- and target specific functionality testing (e.g., target binding, signaling, cell-based assays). Learn more about functional bioassays.
At FyoniBio, we have long-term experiences in the characterization of proteins with a broad spectrum of analytical methods. Contact us to discuss the specific needs of your project and how we can support you.
To enable a fast and accurate process transfer a technology transfer report is written including all information about the developed processes. Technology transfer reports can be directly delivered to a selected CMO. FyoniBio offers additional services to support the process transfer by implementation of process transfer meetings and direct on-site process support.
Successful tech transfer processes have been performed to different GMP sites around the world.
For more relevant information and additional FAQs check also the FAQ section in our resource center.
Process Development Services at FyoniBio – Related Content
Explore our broad spectrum of bioanalytical services which can complement the USP and DSP process development.