
Oligonucleotide Analysis
Comprehensive
Bioanalytical Solutions for Oligonucleotide Therapeutics
Oligonucleotide therapeutics have emerged as a promising class of drugs for treating various diseases, including genetic disorders and certain types of cancer. To ensure the safety and efficacy of these therapeutic agents, precise pharmacokinetic and immunogenicity assessments are crucial in both preclinical and clinical studies. At FyoniBio, you will get comprehensive GCLP-compliant services for oligonucleotide bioanalysis to support the preclinical and clinical development of your oligonucleotide therapeutic. Our services range from oligonucleotide assay establishment and validation to routine sample bioanalysis, including the quality control of critical reagents.
FyoniBio’s bioanalytics experts work with the following types of oligonucleotide therapeutics:
- Antisense oligonucleotides (ASO)
- Small interfering RNA (siRNA)
Customised Assays for Pharmacokinetics (PK) and Anti-Drug Antibodies (ADA)
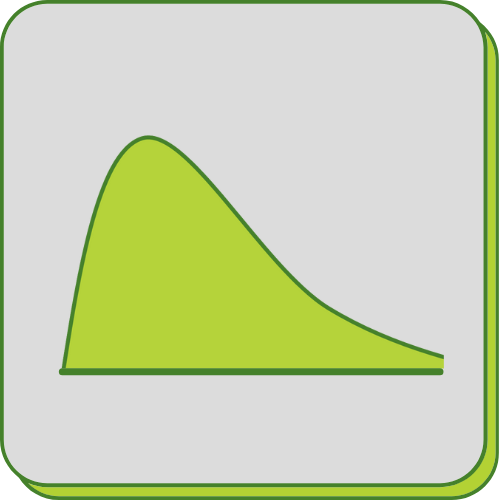
Quantitative assays for pharmacokinetic profiling (PK)
Different assays for immunogenicity assessment (ADA)
Customised assays are pivotal in providing accurate and specific data tailored to the unique characteristics of oligonucleotide therapeutics. Our oligonucleotide assays enable very sensitive and specific quantification of oligonucleotides with any modification, as well as the detection of anti-drug antibodies observed in patients treated with oligonucleotide drugs.
Quantitative Assays to Support Pharmacokinetic Profiling
Pharmacokinetics involves studying the absorption, distribution, metabolism, and excretion (ADME) of drugs within the body. For oligonucleotide therapeutics, specialized assays are required to assess their biodistribution, stability, and clearance.
FyoniBio developed a novel, highly sensitive hybridization electrochemiluminescence immunoassay (hECLIA) to quantify antisense oligonucleotides and other oligonucleotide drugs in a broad range of biological matrices from different species such as serum or plasma, urine, cerebrospinal fluid (CSF), and various tissues (such as liver, heart, muscle, and brain).
This hECLIA method offers several advantages compared to other methods, including:
- Less complex sample preparation and higher sensitivity and throughput rates compared to mass spectrometry.
- Simpler method development, more robust performance, and less interference of modifications to the analytes compared to PCR-based methods.
- Excellent precision and accuracy, even in the low pM range.
- Specific and robust quantification of terminally modified and single- and double-stranded analytes.
No extra sample preparation (other than tissue lysis) and simple and rapid assay execution, allowing for high-throughput application.
Anti-Drug Antibody Assays
Anti-Drug Antibodies (ADAs) can develop in response to oligonucleotide therapeutics, potentially impacting their safety and efficacy. ADA assays are essential for monitoring and mitigating immunogenicity risks. Our ADA services include:
- Assessment of anti-drug antibodies (ADA) in preclinical and clinical samples.
- A three-tiered approach including screening, confirmatory, and titration assay.
- Extended characterization of ADA, including NAb, isotype, and domain specificity.
- Generation of positive control antibodies.
Quality Control
Quality control is critical in bioanalytical services for oligonucleotide therapeutics to ensure the reliability and accuracy of data throughout the project. It safeguards the identity, integrity, and purity of the analyte in biological matrices and critical reagents to perform the assays. Quality control at FyoniBio includes:
- LC-MS based oligonucleotide analysis for identity, integrity, and purity.
- Long-term monitoring of critical reagents of oligonucleotide-based assays.
- Applicability for formulation and stability studies.
Turnaround Time for Oligonucleotide Bioanalysis Projects
FyoniBio has extensive expertise in providing reliable support, demonstrating agility and flexibility to meet our customers’ specific needs. We are committed to delivering high-quality data within competitive turnaround times. Our optimized processes ensure that your oligonucleotide bioanalysis projects are efficiently managed without compromising scientific excellence.
Get in touch with us, we will be happy to support you throughout your clinical development program.
Need more information about our preclinical and clinical custom assay development for your oligonucleotide therapeutic?
Frequently Asked Questions (FAQ) for Oligonucleotide Analysis
ECLIA stands for “ElectroChemiLuminescence ImmunoAssay.” It is a specific type of plate-based immunoassay used in medical diagnostics, particularly in clinical laboratories, to measure certain substances in blood, or other body fluids, and tissues. Similar to the ELISA, antibodies and antigens are routinely employed to detect the presence of specific molecules such as hormones, proteins, nucleic acids, or markers for diseases. Detection occurs through the chemical reaction of the substrate reagent. The result of this reaction is a light signal (luminescence), which can be captured and quantified by the detector on the measuring device. Compared with ELISA the ECLIA technology results in more sensitive, precise, and reliable quantification of these substances in a wider assay range.
Oligonucleotide drugs work by modulating gene expression at the nucleotide level, often by binding to RNA or DNA to affect transcription or translation. Unlike small molecules or biologics that can target proteins or other cellular components, oligonucleotides are characterized by their high targeting accuracy, allowing precise control over gene expression. These drugs are often developed for diseases with a genetic component, where modulation of gene expression offers therapeutic benefits. However, they face specific delivery challenges due to their size and susceptibility to degradation. The oligonucleotide drug innovation and development landscape are comparatively new and is characterized by continuous progress in designing more efficient and targeted therapies, in contrast to other therapeutics that often have longer development histories and established pathways.
For more relevant information and additional FAQs check also the FAQ section in our resource center.
Oligonucleotide Analysis at FyoniBio – Related Content
Watch our latest videos explaining the basics of immunogenicity related to biotherapeutic drug application.
Have a look at the relevant guidelines describing the principles of bioanalytical method validation.
